Infectious diseases have a major impact on the welfare and productivity of animals raised for food production worldwide. Furthermore, poor productivity leads to increased carbon emissions, and the drugs used to control infections can harm the environment and reduce biodiversity. Therefore, better control of infections in food producing animals will play a key role in achieving important policy goals, including Net-Zero and Biodiversity targets.
Understanding how pathogens interact with their host to cause disease is important as it can lead to the development of new methods for disease control. However, this can be technically challenging – not least because these interactions take place inside the animal and out of sight of the investigator! To address this, we are developing “organoids”, which are mini-organs from livestock, grown in laboratory petri dishes, to study host-pathogen interactions at microscopic and molecular levels. The technology should enable us to identify pathogen proteins that are essential for infection and to apply this knowledge in the development of novel vaccines.
Stage
Directory of Expertise
Purpose
Improvements in animal health and welfare are key components in the drive for increased sustainability in livestock agriculture. Disease-free animals mean higher efficiency, which in turn leads to lower carbon emissions, better land use and improved biodiversity. Healthier animals lead to healthier people through the reduced use of antibiotics in animals, slowing down the selection of antibiotic resistant bacteria, and fewer infections transmitted from animals to humans.
Designing new ways of controlling diseases requires an improved understanding of how pathogens (viruses, bacteria, fungi and parasites) are able to infect their host and cause disease. Hosts and pathogens interact on a microscopic and molecular level and therefore investigating this battleground within a live animal is challenging. A desire to address this challenge fueled our development of livestock-derived “organoids” - miniature lab-grown organ tissues. Organoids mimic important features of the tissue they represent, in particular the variety of cell types present and the three-dimensional structures they form, making them functionally similar to real organs.
In the lab organoids are used as experimental disease models, where we infect them with a pathogen or expose them to pathogen molecules in a systematic and controlled manner. This allows us to define important cellular and molecular interactions taking place during an infection and identify pathogen molecules essential for successful infections that can be targeted by novel drugs and/or vaccines - importantly, using organoids in research reduces the need for live animal experiments.
Results
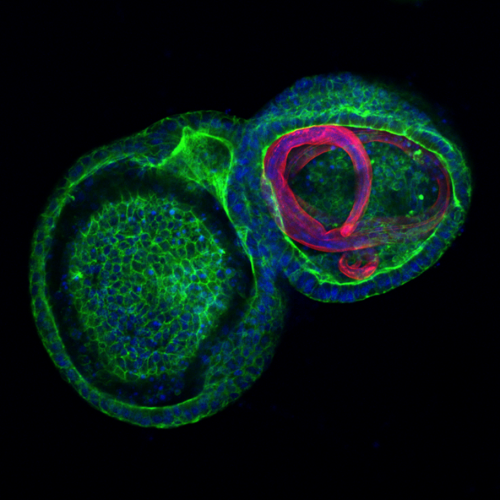
Our research began by growing organoids and demonstrating they are valid models for studying diseases in livestock. Initially, we collected cells from gut tissue from sheep and cultured them in a three-dimensional gel matrix in the presence of growth factors. Under these conditions, the cells became organised into three-dimensional structures (organoids) that closely resemble glandular structures within normal sheep gut. The organoids were then analyzed by microscopy and molecular tools to demonstrate the presence of specialised cell types that we would expect to see in the original animal organ.
Image: Bovine intestinal organoids (mini-guts) viewed down a microscope.
This work revealed that organoids were representative of the tissue from which they were derived, and therefore a valid model for studying disease in livestock and we have since expanded our work to create organoids from cattle, pigs, and horses, making SEFARI scientists world-leading in this technology. Moreover, we are now developing livestock organoids from other tissues that represent key sites of infection, including the lung, mammary gland and liver. We are also using different organoid cultures to model interactions that occur between the host tissues and pathogens ranging from parasitic worms in the guts of sheep and cattle to bacterial infections of the ruminant mammary gland and intestines and viral infections of the lung. Through our continued development and application of species- and tissue-specific organoids in the context of certain livestock diseases, it is our aim to translate new knowledge of host:pathogen interactions into novel disease interventions, such as vaccines. Specifically, we are actively applying our organoid models to a range of particularly pressing and economically important parasitic, bacterial and viral diseases of livestock, including bovine and ovine parasitic gastro-enteritis caused by gastro-intestinal nematodes (GINs); Johne’s disease in sheep and cattle, caused by Mycobacterium avium subsp. paratuberculosis (MAP); Ovine Pulmonary Adenocarcinoma (OPA) in sheep, caused by Jaagsiekte sheep retrovirus (JSRV); mastitis in cattle and sheep and liver fluke infection in cattle and sheep.
Two posters have been produced (alongside the research papers listed below). Further poster details are available on request from the research team.
Image: Communications poster ‘Different veterinary organoid models being developed and applied for investigating infectious diseases at The Moredun Research Institute’.
Image: Communications poster ‘Lab grown, stem cell-derived miniature organs (organoids) recapitulate specific tissues and are a cutting-edge tool for the investigation of diverse and widespread infectious diseases’.
Benefits
The tissue and species specificity retained in organoids provides a valuable culture system for experimentally modelling livestock host:pathogen interactions with detail and precision. We can model aspects of infection dynamics not previously possible with conventional cell cultures.
The tissue- and species-specific nature of livestock organoids is also reducing our reliance on live animal research to address particular research questions.
Furthermore, by developing and using these organoids to model different infections, we are enhancing our understanding of how pathogens interact with their livestock hosts, leading to new ways to prevent and treat diseases, such as new vaccines.
Ultimately, it is hoped this research will help to control diseases in livestock, and in turn contribute to increasing productivity, reducing the environmental impact of farming, ensuring food security, and making livestock agriculture more sustainable.