Antibiotic usage
Globally, antimicrobial resistant infections contribute to around 700,000 deaths annually, whilst in the UK it is estimated that around 7600 deaths each year can be attributed directly to antibiotic-resistant infections (and in Scotland this is around 1500 deaths per annum). Moreover, in the UK over 35,000 deaths annually are indirectly related to multidrug resistant infections. The use and misuse of antibiotics is a major driver of antimicrobial resistance with more than 700 tonnes of antibiotics being consumed in the UK annually, of which people consume 68% and animals consume 32%. In the past, particularly around the 1970s, certain antibiotics, such as ionophores, were widely used as animal growth promoters. Nowadays, the use of antibiotics is closely monitored in agricultural settings in the UK and Scotland which has resulted in a marked reduction in the use of antibiotics on farms. This is due to legislation in the UK (and EU) which bans the routine use of antibiotics in farm animals, restricting prophylactic use other than under exceptional circumstances, and only permits their use to treat disease.
Following the discovery of penicillin in 1928, by the Scottish physician and microbiologist Alexander Fleming, antibiotics have been used to save millions of lives. Antibiotics are estimated to have increased the average human lifespan by approximately 23 years. Due to the emergence of resistance, some antibiotics are no longer effective in treating some diseases. Hence, it continues to be crucial that companies develop new antibiotics and/or therapies to combat the growing threat of antibiotic resistance.
Developing novel antibiotics is however a complex and challenging process. It typically takes over 10 years to get a new antibiotic to market and incurs considerable cost. In the past five years, or so, there have only been around one dozen new antibiotics that have entered the market. One notable example, Cifidericol, can target a wide range of pathogens, including those deemed critical by the World Health Organisation (WHO). The slow development of new antibiotics onto the market means that there is a pressing need for more innovative solutions to combat antibiotic resistance particularly in pathogens.
Critical pathogens of concern
The six leading pathogens, according to the World Health Organisation (WHO), associated with antibiotic resistance are Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species which are referred to as the ESKAPE pathogens. These bacterial pathogens can be resistant to commonly used antibiotics which makes them a major concern for hospital-acquired infections. This is in part due to each having developed, or acquired, various mechanisms to resist multiple commonly used antibiotics, which complicates treatment and increases the risk of severe, difficult-to-treat infections.
Other pathogens of concern include zoonotic food poisoning pathogens such as Escherichia coli, Salmonella enterica, Campylobacter jejuni, Clostridium perfringens, and Clostridioides difficile. These are mainly considered to be zoonotic pathogens meaning that they can pass through direct contact with animals, indirectly from animal surroundings or through food-borne transmission (consuming contaminated food or water). Hence there is a pressing need to prevent and control outbreaks particularly in farm settings where humans and animals can be in close contact.
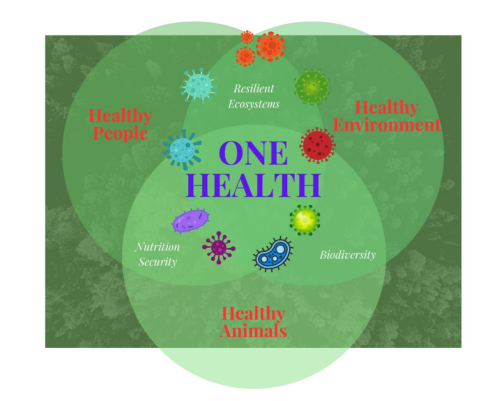
One Health approach: Probiotics for humans, animals and plants
Scotland follows a “One Health” approach, recognising the interconnectedness of human, animal and environmental health. The Scottish One Health Antimicrobial use and Antimicrobial Resistance (SONAAR) program monitors trends in antibiotic use and resistance to inform interventions with the aim of reducing the burden of infection and antimicrobial resistance and therefore benefit One Health.
One health approaches using beneficial microbes to reduce non-essential use of antibiotics and chemical pesticides (Credit; authors)
Alternative approaches that are being used to tackle multi-drug-resistant bacteria include the development of new probiotics (and/or biotherapeutic agents) to offer targeted and effective benefits. These approaches can be tailored to include identifying specific bacteria possessing potent inhibitory activities against pathogens of concern, which do not themselves possess antimicrobial resistance traits. A few environmental bacterial strains have already been approved by the European Food Safety Authority (EFSA) for use in animal feed to promote animal growth and are therefore good alternatives to antibiotic-based growth promoters.
The application of bacteria with plant growth promoting features, so-called plant probiotics, represents a sustainable solution to increase crop production without application of chemical fertilisers and pesticides while producing better-quality products. Such bacteria can benefit plant nutrient uptake, promote plant growth, and prevent plant diseases caused by plant pathogens. Plant probiotics, used for biocontrol and biofertilisers, may be formulated with single bacterial strains or with mixtures of carefully selected isolates (ideally, by using native soil organisms). The latter helps to combine different beneficial effects, whilst delivering tangible solutions to feed the world while at the same time protecting ecosystems and improving food quality. Due to bacterial strain variation, it is important to carefully consider the merits of individual strains identified for use in human, plant and animal feed and ensure that these strains are not carrying antibiotic resistance genes or virulence traits which can be studied by mining bacterial genomes for such traits.
Initial research at the Rowett Institute, which includes mining complete bacterial genomes of Campylobacter strains for tetracycline resistance genes and phenotypes which revealed that in this pathogen, this trait is mainly conferred by ribosome protection proteins. Moreover, screening isolates within our bacterial culture collection, has revealed promising candidate strains that could be effective probiotics for farm animals with the ability to inhibit the growth of pathogens.
Screening bacterial isolates for ability to inhibit pathogens (Credit: authors)
There are several examples of related bacterial strains, isolated from environmental samples, being used for this purpose that have been approved for use by the European Food Safety Agency (EFSA). Our current research therefore provides early exciting findings in combating antimicrobial resistance (AMR), which is one of the few challenges that unites global interests and concerns for human and animal health. In addition, this will help to provide security for the food and agricultural sectors, in Scotland, the UK and globally.
Written by Dr Sylvia H. Duncan Prof Karen Scott and Dr Madalina Neacsu, the Rowett Institute.