Importance of farmed animals to Scotland
Agriculture is a critically important industry for Scotland, contributing £2.9 billion to the economy each year, and is responsible for much of the £5 billion in food and drinks that Scotland exports annually. The sector employed 67,400 people in 2024, further underpinning its importance to Scotland’s economy. Scottish agriculture is also essential for food security. Currently, the UK is only 60% self-sufficient in food, with Scotland making an important contribution to the output, despite accounting for only 8% of the population.
Key diseases that impact on the health of farmed animals in Scotland
Infectious diseases of farmed animals are significant threats to agriculture, and are estimated to cost the UK livestock sector between £290 million and £710 million per year due to a combination of veterinary and treatment costs, early animal mortality, and reduced yield. In addition to this financial cost, infectious diseases also result in significant animal suffering and also endanger human health, since diseases can be transmitted to humans when animal products are consumed.
Two of the main types of infectious disease that impact livestock are gastroenteritis and mastitis. Gastroenteritis refers to inflammation of the intestinal tract, with resulting symptoms such as diarrhoea. Notable gastroenteritis pathogens that infect farmed animals in Scotland include Escherichia coli, Campylobacter and Salmonella species, all of which can spread to humans and are common causes of food poisoning. Mastitis refers to inflammation of mammary glands and udder tissue. It is one of the most economically significant diseases in the dairy industry, leading to reduced milk production, increased veterinary costs and premature culling of affected animals. Up to 12% of cattle can be affected by mastitis per year, and this is estimated to cost the industry between 6.7% and 10.2% of total annual worth.
Management and control of gastroenteritis and mastitis
Typically, conditions like gastroenteritis and mastitis are treated with antibiotics. However, overuse of antibiotics has seen the emergence of pathogens with resistance against these drugs, rendering them less effective. Importantly, genes conferring resistance to antibiotics can also readily spread to other bacteria, and the recipient microbes then become resistant to antibiotics themselves and become harder to treat. This spread of antimicrobial resistance is contributing to a worsening global public health crisis, which now results in millions of deaths around the world each year due to untreatable infections.
In recognition of these concerns, the EU recently introduced strict regulations to reduce routine antibiotic use in livestock. In the UK, although there have been voluntary reductions in antibiotic use, by about 50% over the past decade, there is increasing pressure from health organisations and the public to reduce antibiotic use even further in agriculture, emphasising the need for alternative animal husbandry practices. The challenge lies in balancing the health of farmed animals with the need to protect public health from the risks associated with antibiotic resistance.
New solutions to the problem of infectious diseases in livestock
It is clear that alternative strategies must be developed to protect livestock against infectious diseases, allowing us to reduce our dependence on antibiotics. One hugely promising approach is to harness the communities of beneficial microbes (collectively termed “microbiotas”) that live within livestock animals. It is well established that these beneficial microbes play many key roles in animal health, with one of the major benefits being that they can actively suppress the growth of invading pathogens, and reduce their ability to colonise, replicate and cause disease. Manipulating the livestock microbiota in order to strengthen this defence therefore offers significant promise as a novel approach to protect against infectious diseases. Maintaining a healthy microbiota is also important for nutrient absorption, enhancing gut barrier function, and promoting the host animal’s immune system. This can result in improved animal growth and feed efficiency, and can enhance the resulting quality of meat, milk and eggs.
Next-generation probiotics
It is possible to alter the microbiota by supplementing the diet that is fed to livestock, including by introducing beneficial microbes in the form of probiotics. Traditional probiotic formulations typically include species such as Lactobacillus and Bifidobacterium strains, but evidence for their effectiveness has so far proven to be mixed. There is therefore increasing interest in developing next-generation probiotics derived from livestock microbiotas, as well as novel microorganisms from environments such as soil. However, the microbiotas that inhabit livestock animals and soil are highly complex, being comprised of many thousands of different microbial species. Currently, it is largely unknown which individual species have the greatest ability to inhibit the growth of key pathogens that cause infectious diseases in farmed animals.
We have begun to address this knowledge gap in our Scottish Government-funded research at the Rowett Institute, University of Aberdeen. In our work to date, we have identified a number of livestock and soil-derived bacteria with potent antimicrobial activity against a range of important livestock pathogens that cause gastroenteritis and mastitis (Figure 1). These early findings are extremely promising, and we are currently further characterising these beneficial microbes, before testing them in pre-clinical models of livestock guts. Our eventual goal is to develop our most suitable strains as potential next-generation probiotics that could be fed to livestock in order to bolster their protection against infectious diseases.

Figure 1. Illustrative results showing livestock-derived bacteria inhibiting the gastroenteritis-causing pathogen Campylobacter jejuni. Livestock bacteria are numbered spots, with clear zones surrounding spots showing where growth of the pathogen has been inhibited. Species in spots 10 and 11 showed particularly strong inhibition in this example.
Conclusions
Next-generation probiotics offer a promising avenue for future sustainable livestock farming (Figure 2), addressing both productivity and animal health concerns without relying so heavily on antibiotics. Microbiota research is currently one of the hottest topics in global science and, as research progresses, we can expect to see more innovative and precision probiotic solutions tailored for various livestock species in order to reduce incidence, carriage and transmission of infectious disease. These strategies could also help to combat antimicrobial resistance, which is a challenge of global importance, impacting both human and animal health. Furthermore, they can help to increase profitability and robustness for the food and agricultural sectors, in Scotland, the UK and globally.
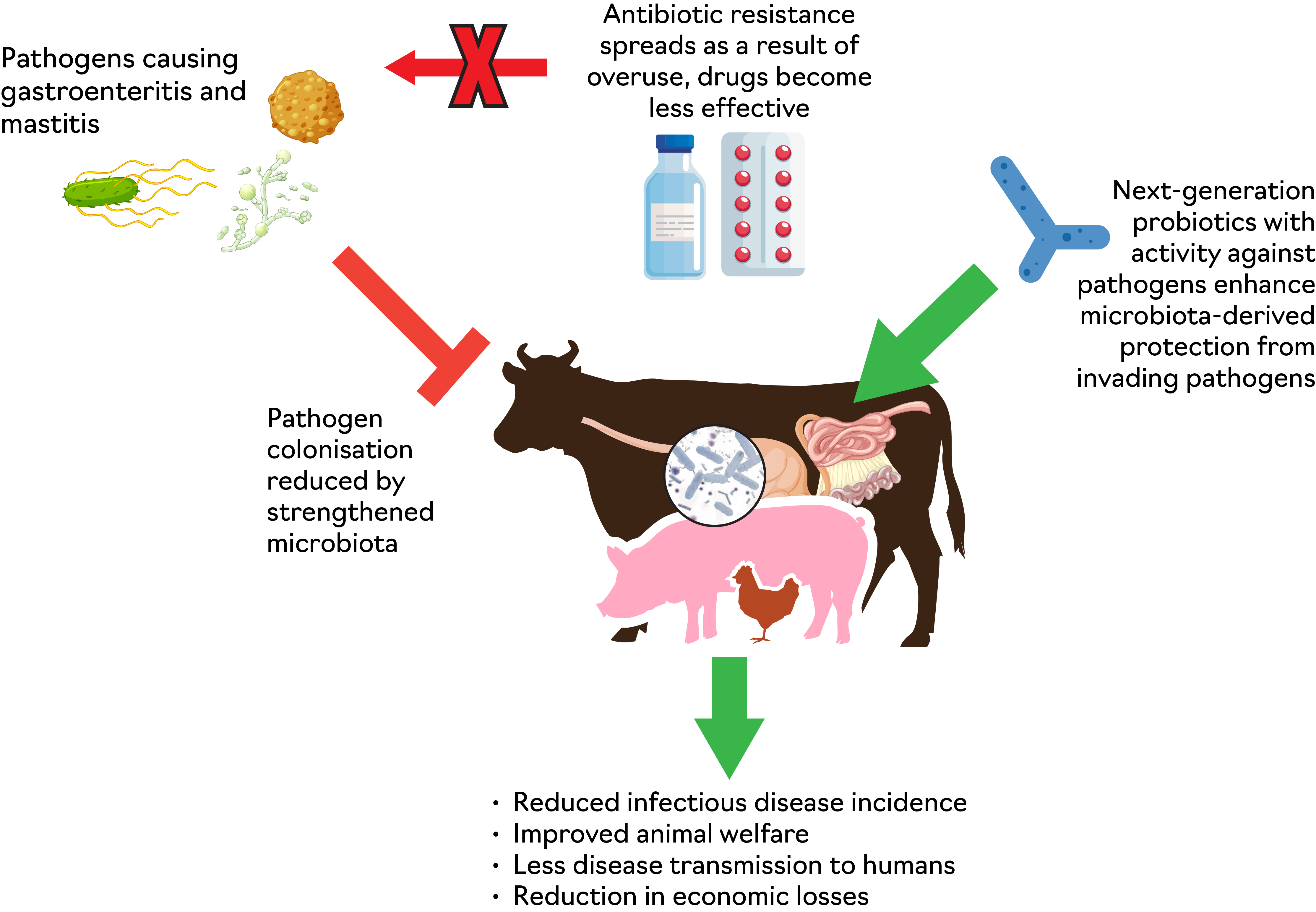
Figure 2. Schematic diagram showing the promise of novel microbiota-targeted therapies to reduce infectious disease in livestock
Prof Alan Walker and Dr Sylvia H. Duncan at the Rowett Institute.
